Advertisements
Advertisements
Question
Explain the Structural isomerism term with example.
Explain
Solution
It is a phenomenon in which the compounds having different structural formulas have the same molecular formula.
E.g. n-Butane and isobutane are structural isomers as they have different structural formulae but have a same molecular formula: C4H10.
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\\end{array}\]
n-Butane (C4H10)
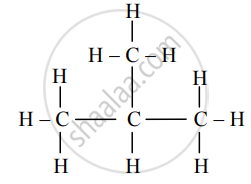
Isobutane (C4H10)
shaalaa.com
Is there an error in this question or solution?
