Advertisements
Advertisements
Question
CH3–CH2–CHO : propanal : : CH3–COOH : _______
Solution
CH3–CH2–CHO : propanal : : CH3–COOH : ethanoic acid
APPEARS IN
RELATED QUESTIONS
While studying saponification reaction for the preparation of soap, a teacher suggested to a student to add a small quantity of common salt to the reaction mixture. The function of common salt in this reaction is to
(A) reduce the alkalinity of the soap
(B) reduce the acidity of the soap
(C) enhance the cleansing capacity of soap
(D) favour precipitation of soap
How esters are prepared ?
Identify the term or substance based on the descriptions given below
Ice like crystals formed on cooling an organic acid sufficiently.
Give a reason for Conductivity of dilute hydrochloric acid is greater than that of acetic acid
Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 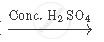
How would you distinguish experimentally between an alcohol and carboxylic acid on the basis of a chemical property?
What type to compound is CH3COOH?
Consider the following organic compounds:
HCHO, C2H5OH, C2H6, CH3COOH, C2H5CI
Choose two compounds which can react in the presence of conc. H2SO4 to form an ester. Give the name and formula of the ester formed.
An organic compound A (molecular formula C2H4O2) reacts with Na metal to form a compound B and evolves a gas which burns with a pop sound. Compound A on treatment with an alcohol C in the presence of a little of concentrated sulphuric acid forms a sweet-smelling compound D (molecular formula C3H6O2). Compound D on treatment with NaOH solution gives back B and C. Identify A, B, C and
A student takes Na2CO3 powder in a test tube and pours some drops of acetic acid over it. He observes:
(A) no reaction in the test tube
(B) colourless gas with pungent smell
(C) bubbles of a colourless and odourless gas
(D) white fumes with smell of vinegar
Explain the following term with example.
Monomer
How will you carry out the following conversions?
Ethene to acetic acid
How Will You Carry Out the Following Conversions?
Ehane to acitic acid
Fill in the blank with appropriate word/words.
Esterification is the reaction between carboxylic acid and ______ in presence of ____
State how the following conversions can be carried out:
Ethyl chloride to Ethene.
State how the following conversions can be carried out:
Ethene to Ethyl alcohol
State are relevant observations for following reactant:
Addition of ethyl alcohol to acetic acid in presence of conc. H2SO4
Write the molecular formula of the given compound.
Sodium ethanoate
Explain the following reaction with an example.
Esterification
