Advertisements
Advertisements
Question
State how the following conversions can be carried out:
Ethyl chloride to Ethene.
Solution
Ethyl chloride, treated with alcoholic KOH, gives Ethene.
\[\ce{\underset{chloride}{\underset{Ethyl}{C2H5Cl}} + KOH_{(aq)} -> \underset{Ethanol}{C2H5OH} + KCl}\]
APPEARS IN
RELATED QUESTIONS
Give the name and structural formula of one homologue of HCOOH.
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 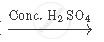
Consider the following organic compounds:
HCHO, C2H5OH, C2H6, CH3COOH, C2H5CI
Choose two compounds which can react in the presence of conc. H2SO4 to form an ester. Give the name and formula of the ester formed.
An organic compound A (molecular formula C2H4O2) reacts with Na metal to form a compound B and evolves a gas which burns with a pop sound. Compound A on treatment with an alcohol C in the presence of a little of concentrated sulphuric acid forms a sweet-smelling compound D (molecular formula C3H6O2). Compound D on treatment with NaOH solution gives back B and C. Identify A, B, C and
Name the product formed and give an appropriate chemical equation for the following:
Ethanol oxidised by acidified potassium dichromate?
Why is Soda lime used not only NaOH?
Write three physical properties of acetic acid.
Draw the structure formula of ethyne.
Write the chemical equation for the following:
Saponification Reaction
A spatula full of sodium carbonate is taken in a test tube and 2 mL of dilute ethanoic acid is added to it.
Suggest a method of testing the gas liberated in the reaction.
