Advertisements
Advertisements
Question
State how the following conversions can be carried out:
Ethene to Ethyl alcohol
Solution 1
Ethene adds a molecule of water in the presence of mineral acids to form ethyl alcohol.
\[\ce{CH2 = CH2 + H2O ->[H+] CH3 - CH2 - OH}\]
Solution 2
\[\ce{\underset{ethene}{CH2 = CH2} + H2SO4 -> \underset{hydrogen sulphate}{\underset{ethyl}{C2H5HSO4}} -> \underset{ethanol}{C2H5OH}\underset{Sulphuric acid}{H2SO4}}\]
APPEARS IN
RELATED QUESTIONS
Write the IUPAC names, common names and formulae of the first two members of the homologous series of carboxylic acids.
What happens when ethanoic acid reacts with sodium carbonate? Write chemical equation of the reaction involved.
How does ethanoic acid react with sodium hydrogen carbonate? Give equation of the reaction which takes place.
Esters are sweet-smelling substances and are used in making perfumes. Describe an activity for the preparation of an ester with the help of a well labelled diagram. Write an equation for the chemical reaction involved in the formation of the ester. Also write the names of all the substances involved in the process of esterification.
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is most likely to be sweet-smelling?
On adding NaHCO3 to acetic acid, a gas is evolved which turns lime water milky due to the formation of:
(1) Calcium bicarbonate
(2) Calcium hydroxide
(3) Calcium carbonate
(4) Calcium acetate
Write a balanced equation for the following:
Write the equation for the preparation of ethylene from ethyl alcohol.
Why is pure acetic acid also known as glacial acetic acid?
Draw the structure formula of ethyne.
Observe the diagram given below and answer the questions:
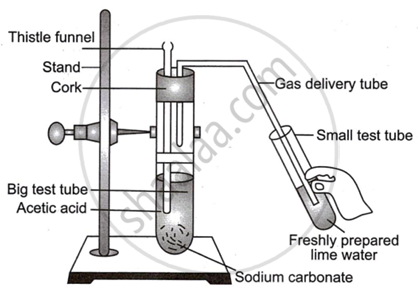
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
