Advertisements
Advertisements
Question
A spatula full of sodium carbonate is taken in a test tube and 2 mL of dilute ethanoic acid is added to it.
Suggest a method of testing the gas liberated in the reaction.
Solution
To test the gas liberated in the reaction between sodium carbonate and dilute ethanoic acid, carbon dioxide (CO₂) passed through lime water (calcium hydroxide solution). If carbon dioxide is present, it reacts with the lime water to produce calcium carbonate, a white precipitate that turns the lime water milky. The change indicates the existence of carbon dioxide. The chemical reaction for this test is as follows:
\[\ce{CO2(g) + Ca(OH)2(aq) -> CaCO3(s) + H2O(l)}\]
This method effectively demonstrates the presence of carbon dioxide gas evolved during the reaction.
APPEARS IN
RELATED QUESTIONS
A student adds a spoon full of powdered sodium hydrogen carbonate to a flask containing ethanoic acid. List two main observations, he must note in his note book, about the reaction that takes place. Also write chemical equation foe the reaction.
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 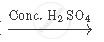
What happens when propanoic acid is warmed with methanol in the presence of a few drops of concentrated sulphuric acid? Write equation of the reaction involved.
How is acetic acid prepared from acetylene?
While studying saponification reactions, the following comments were noted down by the students :
(I) Soap is a salt of fatty acids.
(II) The reaction mixture is basic in nature.
(III) In this reaction heat is absorbed.
(IV) This reaction is not a neutralisation reaction.
Which of these are the correct comments ?
(A) I and III only
(B) I, II and III
(C) II, III and IV
(D) I and II only
Why is pure acetic acid also known as glacial acetic acid?
What type of compound is formed by the reaction between acetic acid and an alcohol?
State how the following conversions can be carried out:
Ethene to Ethyl alcohol
Draw the structural formula for the following:
Ethanoic acid
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
