Advertisements
Advertisements
Question
Explain the following term with example.
Monomer
Solution
Monomer: A monomer is a molecule that forms the basic unit for polymers. They may be considered as building blocks from which proteins are made. Monomers may bind to other monomer unit to form a repeating chain molecule. Monomers may be either natural or synthetic in origin.
For example: Ethylene, vinyl chloride, styrene etc.
APPEARS IN
RELATED QUESTIONS
A student adds a few drops of ethanoic acid to test tubes X, Y and Z containing aqueous solutions of sodium chloride, sodium hydroxide and sodium carbonate, respectively. If he now brings a burning splinter near the mouth of the test tubes immediately after adding ethanoic acid in each one of them, in which of the test tube or test tubes the flame will be extinguished?
(A) X and Y
(B) Y and Z
(C) X and Z
(D) only Z
Write the formulae of methanoic acid.
What type of compound is formed when a carboxylic acid reacts with an alcohol in the presence of conc. H2SO4?
How does ethanoic acid react with sodium hydrogen carbonate? Give equation of the reaction which takes place.
An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogen carbonate. Give the name and formula of X.
Write the IUPAC name of the following:
\[\begin{array}{cc}
\ce{CH3}\\
|\\
\ce{CH3 -C -CH3}\\
|\\
\ce{CH3}
\end{array}\]
Ethanol can be oxidized to ethanoic acid. Write the equation and name the oxidizing agent.
Write the names of three compounds which can be oxidised directly or in stages to produce acetic acid.
Give the structural formulae of acetic acid.
Acetic acid is a typical acid. Write one equation in case of its reactions with a carbonate.
Name the first four members of alphatic carboxylic acid.
Draw the structural formula of a compound with two carbon atoms in the following case:
An alkane with a carbon to carbon single bond.
What type of compound is formed by the reaction between acetic acid and an alcohol?
Write a fully balanced equation for the folowing case:
Acitic acid is warmed with ethanol in the presence of concentrated sulpheric acid.
State the observation
When the gaseous product obtained by dehydration of ethyl alcohol is passed through bromine water.
Identify the term or substance based on the descriptions given below:
Ice like crystals formed on cooling an organic acid sufficiently.
Choose the correct alternative and rewrite the following:
Some acetic acid is treated with solid NaHCO3, the resulting solution will be _________________.
The reaction between acetic acid and sodium carbonate is shown in the following figure.
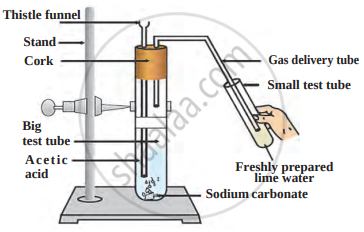
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
A spatula full of sodium carbonate is taken in a test tube and 2 mL of dilute ethanoic acid is added to it.
Suggest a method of testing the gas liberated in the reaction.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
