Advertisements
Advertisements
Question
What type of compound is formed when a carboxylic acid reacts with an alcohol in the presence of conc. H2SO4?
Solution
Esters are formed when carboxylic acid reacts with alcohol in the presence of concentrated sulphuric acid.
APPEARS IN
RELATED QUESTIONS
Complete the following chemical equations : C2H5OH`("Conc."H_2SO_4)/(443K)`>
Why is the conversion of ethanol into ethanoic acid an oxidation reaction?
An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogen carbonate. Give the name and formula of X.
Acetic acid is a typical acid. Write one equation in case of its reactions with a base/alkali?
Name the functional group present in the following compound:
HCOOH
On adding 2 mL acetic acid to 2 mL of water in a test tube, it was observed that
(A) a clear and transparent solution is formed
(B) a white precipitate is formed almost immediately
(C) two separate layers were formed
(D) a colourless and odourless gas is evolved
Explain the following term with example.
Reduction
How is ethyne prepared in the laboratory?
A student while observing the properties of acetic acid would report that this smells like ______.
The reaction between acetic acid and sodium carbonate is shown in the following figure.
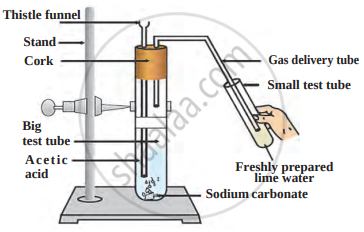
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
