Advertisements
Advertisements
Question
On adding 2 mL acetic acid to 2 mL of water in a test tube, it was observed that
(A) a clear and transparent solution is formed
(B) a white precipitate is formed almost immediately
(C) two separate layers were formed
(D) a colourless and odourless gas is evolved
Solution
Acetic acid is colourless and dissolves completely in water. Hence, when 2 ml of acetic acid is added to 2 ml of water a clear and transparent solution is formed.
The Correct Answer is A
APPEARS IN
RELATED QUESTIONS
Give balanced chemical equations for Sodium ethanoate to methane.
Esters are sweet-smelling substances and are used in making perfumes. Describe an activity for the preparation of an ester with the help of a well labelled diagram. Write an equation for the chemical reaction involved in the formation of the ester. Also write the names of all the substances involved in the process of esterification.
Consider the following organic compounds:
HCHO, C2H5OH, C2H6, CH3COOH, C2H5CI
Choose two compounds which can react in the presence of conc. H2SO4 to form an ester. Give the name and formula of the ester formed.
What do you observe when acetic acid is added to ethyl alcohol in the presence of sulphuric acid?
While studying saponification reactions, the following comments were noted down by the students :
(I) Soap is a salt of fatty acids.
(II) The reaction mixture is basic in nature.
(III) In this reaction heat is absorbed.
(IV) This reaction is not a neutralisation reaction.
Which of these are the correct comments ?
(A) I and III only
(B) I, II and III
(C) II, III and IV
(D) I and II only
A student adds 4 mL of acetic acid to a test tube containing 4 mL of distilled water. He then shakes the test tube and leaves it to settle. After about 10 minutes he observes:
(A) a layer of water over the layer of acetic acid
(B) a layer of acetic acid over the layer of water
(C) a precipitate settling at the bottom of the test tube
(D) a clear colourless solution
State how the following conversions can be carried out:
Ethene to Ethyl alcohol
Which of these is not an organic acid?
The reaction between acetic acid and sodium carbonate is shown in the following figure.
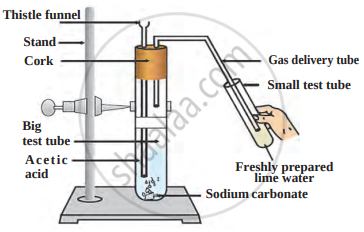
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
Write the chemical equation for the following:
Saponification Reaction
