Advertisements
Advertisements
Question
Draw the electron-dot structure of NH3 and state the type of bonding.
Solution
The electron-dot structure of NH3 is:
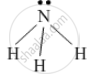
In NH3, each hydrogen atom shares its one electron with the nitrogen atom to form a covalent bond.
APPEARS IN
RELATED QUESTIONS
State the reason to explain why covalent compounds "are bad conductors of electricity".
What is buckminsterfullerene?
Draw the electron-dot structure of N2 and state the type of bonding.
What is graphite?
The solution of one of the following compounds will not conduct electricity. This compounds is:
(a) NaCl
(b) CCl4
(c) MgCl2
(d) CaCl2
The electronic configurations of two elements A and B are given below:
| A | 2, 6 |
| B | 2, 8, 1 |
(a) What type of chemical bond is formed between the two atoms of A?
(b) What type of chemical bond will be formed between the atoms of A and B?
An element E exists in three allotropic forms A, B and C. In allotrope A, the atoms of element E are joined to form spherical molecules. In allotrope B, each atom of element E is surrounded by three other E atoms to form a sheet like structure. In allotrope C, each atom of element E is surrounded by four other E atoms to form a rigid structure.
(a) Name the element E.
(b) What is allotrope A.
(c) What is allotrope B?
(d) What is allotrope C?
(e) Which allotrope is used in making jewellery?
(f) Which allotrope is used in making anode of a dry cell?
What is the difference between ionic compounds and polar covalent compounds?
State the type of bonding in the following molecule.
Water
Write answer as directed.
What causes the existance of very large number of carbon compound ?
Explain the following:
Covalent compounds have low melting and boiling point.
Electrons are getting added to an element Y:
Is Y getting oxidised or reduced?
What are the term defined below?
A bond formed by share pair of electrons, each bonding atom contributing one electron to the pair.
State the type of bond formed when the combining atom has zero E.N. difference.
Write the molecular formula of the given compound.
Acetic acid
Write a short note.
Characteristics of carbon
Which of the following statements are usually correct for carbon compounds? These
- are good conductors of electricity
- are poor conductors of electricity
- have strong forces of attraction between their molecules
- do not have strong forces of attraction between their molecules.
An element A is soft and can be cut with a knife. This is very reactive to air and cannot be kept open in air. It reacts vigorously with water. Identify the element from the following
Although metals form basic oxides, which of the following metals form an amphoteric oxide?
In covalent bond formation, the sharing of ______ electrons takes place in their outermost shell.
