Advertisements
Advertisements
Question
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
ethyne
Solution
Structure of ethyne (C2H2) is as follows:
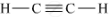
Ethyne has one triple bond between its carbon atoms, and two single bonds between its carbon and hydrogen atoms.
APPEARS IN
RELATED QUESTIONS
‘A’ is an element having four electrons in its outermost orbit. An allotropes ‘B’ of this element is used as a dry lubricant in machinery and in pencil leads. So:
i. Write the name of element ‘A’ and its allotropes.
ii. State whether ‘B’ is a good conductor or non-conductor of electricity?
Define Unsaturated hydrocarbon.
Define Catenation.
Fill in the following blank with suitable word:
The form of carbon which is used as a lubricant at high temperature is .........
Give the names and structural formulae of one saturated cyclic hydrocarbon and one unsaturated cyclic hydrocarbon.
Answer the following question.
Name a cyclic unsaturated carbon compound.
The name of the compound CH3 — CH2 — CHO is
Name the functional groups present in the following compounds
- CH3 CO CH2 CH2 CH2 CH3
- CH3 CH2 CH2 COOH
- CH3 CH2 CH2 CH2 CHO
- CH3 CH2 OH
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.....}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} ≡ \ce{C} - \ce{H}\\
\phantom{}|\phantom{....}|\phantom{..........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{..........}\\
\end{array}\]
Write the name of the following compound
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{}\\
\ce{H - C - C - C - C - C - C - C = O}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{.....}\\
\phantom{.}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}
\end{array}\]
