Advertisements
Advertisements
Question
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
ethene
Solution
Structure of ethene (C2H4) is as follows:
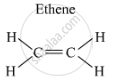
Ethene has a double bond between its carbon atoms, and four single bonds between its carbon and hydrogen atoms.
APPEARS IN
RELATED QUESTIONS
Give any two differences between alkanes and alkenes.
Define Catenation.
Why does carbon form strong bonds with most other elements ?
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Fill in the following blank with suitable word:
Ethyne has ......... carbon-hydrogen single bonds.
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6
(b) C6H12 and C5H12
(c) C4H6 and C6H12
(d) C2H6 and C4H10
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which two formulae represent unsaturated hydrocarbons having triple bonds?
Which of the following hydrocarbons is unsaturated?
C3H4; C2H6
Structural formula of ethyne is ______.
The formulae of four organic compounds are shown below. Choose the correct option
| A | B | C | D |
| \[\begin{array}{cc} \phantom{.}\ce{H}\phantom{......}\ce{H}\\ \phantom{.}\backslash\phantom{.....}/\\ \ce{C = C}\\ /\phantom{.....}\backslash\\ \ce{H}\phantom{......}\ce{H} \end{array}\] |
\[\begin{array}{cc} \phantom{........}\ce{H}\phantom{.....}\ce{O}\\ \phantom{.......}|\phantom{....}//\\ \ce{H - C - C}\\ \phantom{......}|\phantom{.....}\backslash\\ \phantom{...........}\ce{H}\phantom{.....}\ce{O - H} \end{array}\] |
\[\begin{array}{cc}
|
\[\begin{array}{cc} \ce{H}\phantom{...}\ce{H}\phantom{....}\\ |\phantom{....}|\phantom{....}\\ \ce{H - C - C - O - H}\\ |\phantom{....}|\phantom{.....}\\ \ce{H}\phantom{....}\ce{H}\phantom{.....} \end{array}\] |
