Advertisements
Advertisements
Question
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
benzene
Solution
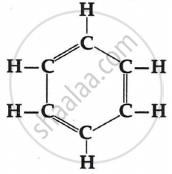
Structure of benzene (C6H6 ) is as follows:
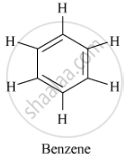
A benzene molecule has three carbon-carbon double bonds, three carbon-carbon single bonds and six carbon-hydrogen single bonds.
APPEARS IN
RELATED QUESTIONS
List two reasons for carbon forming a large number of compounds. Name the type of bonding found in most of its compounds. Why does carbon form compounds mainly by this kind of bonding?
Give reason why the carbon compounds generally have low melting and boiling points.
Fill in the following blank with suitable word:
Compounds of carbon with hydrogen alone are called ..........
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains single bonds as well as a double bond?
You are given the following molecular formulae of some hydrocarbons:
Which formula represents benzene?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae can represent cyclic hydrocarbons?
Write the molecular and structural formula of a cyclic hydrocarbon whose molecule contains 8 atoms of carbon.
Which of the following are correct structural isomers of butane?
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.....}\backslash\phantom{..}|\\
\phantom{.....}\ce{H}\phantom{.......}\ce{C - H}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{....}\phantom{....}|\\
\ce{H}\phantom{........}\ce{H}\\
|\\
\ce{H - C - H}\\
|\\\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}
\end{array}\]
How many electrons are there in the outermost orbit of carbon?
Name the following: