Advertisements
Advertisements
Questions
Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in a molecule of cyclohexane?
Draw the structure of the molecule of cyclohexane (C6H12)·
Solution
The molecular formula of cyclohexane is C6H12.
Its structure is as follows:
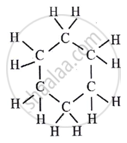
A cyclohexane molecule has six carbon-carbon single bonds and twelve carbon-hydrogen single bonds. Therefore, there are 18 covalent bonds in one molecule of cyclohexane.
Notes
Students should refer to the answer according to their questions.
APPEARS IN
RELATED QUESTIONS
Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential condition for an addition reaction. Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction.
List two reasons for carbon forming a large number of compounds. Name the type of bonding found in most of its compounds. Why does carbon form compounds mainly by this kind of bonding?
Draw the structures for Hexanal.
Give reason why the carbon compounds do not conduct electricity in the molten state.
Write the molecular formula and structure of benzene.
How many isomers of the following hydrocharbons are possible?
C4H10
The hydrocarbon which has alternate single and double bonds arranged in the form of a ring is:
(a) cyclobutane
(b) benzene
(c) butene
(d) hexene
Write the molecular and structural formula of a cyclic hydrocarbon whose molecule contains 8 atoms of carbon.
How will you prove that C4H8 and C5H10 are homologues?
Draw the structure of propanol.
