Advertisements
Advertisements
प्रश्न
Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in a molecule of cyclohexane?
Draw the structure of the molecule of cyclohexane (C6H12)·
उत्तर
The molecular formula of cyclohexane is C6H12.
Its structure is as follows:
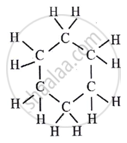
A cyclohexane molecule has six carbon-carbon single bonds and twelve carbon-hydrogen single bonds. Therefore, there are 18 covalent bonds in one molecule of cyclohexane.
Notes
Students should refer to the answer according to their questions.
APPEARS IN
संबंधित प्रश्न
Give reason why the carbon compounds do not conduct electricity in the molten state.
Which of the following is the molecular formula of benzene?
C6H6, C6H10, C6H12, C6H14
Which of the two has a branched chain : isobutane or normal butane?
What is the unique property of carbon atom? How is this property helpful to us?
What are hydrocarbons? Explain with examples.
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains single bonds as well as a double bond?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae can represent cyclic hydrocarbons?
Draw two possible isomers of the compound with molecular formula C3H6O and write their names.
Give the electron dot structures of the above two compounds.
Hydrocarbons are mainly composed of which of these?
How will you prove that C4H8 and C5H10 are homologues?
