Advertisements
Advertisements
प्रश्न
Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in a molecule of cyclohexane?
Draw the structure of the molecule of cyclohexane (C6H12)·
उत्तर
The molecular formula of cyclohexane is C6H12.
Its structure is as follows:
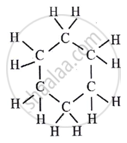
A cyclohexane molecule has six carbon-carbon single bonds and twelve carbon-hydrogen single bonds. Therefore, there are 18 covalent bonds in one molecule of cyclohexane.
Notes
Students should refer to the answer according to their questions.
APPEARS IN
संबंधित प्रश्न
Draw the structures for Butanone
Explain why propane cannot exhibit the structural isomerism property.
Draw the structures of possible isomers of butane, C4H10.
Fill in the following blank with suitable word:
The property of carbon atoms to form long chains in compounds is called ...........
Write the molecular formula and structure of benzene.
What is the molecular formula and structural formula of a cyclic hydrocarbon whose one molecule contains 8 hydrogen atoms?
The functional group which always occurs in the middle of a carbon chain is:
(a) alcohol group
(b) aldehyde group
(c) carboxyl group
(d) ketone group
Pentane has the molecular formula C5H12. It has ______.
Intake of small quantity of methanol can be lethal. Comment.
How many isomers are possible for the compound with the molecular formula C4H8? Draw the electron dot structure of branched-chain isomer.
