Advertisements
Advertisements
प्रश्न
Write two points of difference in the structures of diamond and graphite.
उत्तर
| Sl.No | Structure of Diamond | Structure of Graphite |
| 1. | Each carbon atom is joined to four other carbon atoms by strong covalent bonds to form a regular tetrahedron. | Each carbon atom is joined to three other carbon atoms by strong covalent bonds to form flat hexagonal rings. |
| 2. | Compact and rigid structure (hard) |
Layered structure (soft) |
APPEARS IN
संबंधित प्रश्न
Why covalent compounds are different from ionic compounds?
Explain why, diamond is hard while graphite is soft (though both are made of carbon atoms).
Out of KCl, HCl, CCl4 and NaCl, the compounds which are not ionic are:
(a) KCl and HCl
(b) HCl and CCl4
(c) CCl4 and NaCl
(d) KCl and CCl4
The number of carbon atoms joined in a spherical molecule of buckminsterfullerene is:
(a) fifty
(b) sixty
(c) seventy
(d) ninety
An element E exists in three allotropic forms A, B and C. In allotrope A, the atoms of element E are joined to form spherical molecules. In allotrope B, each atom of element E is surrounded by three other E atoms to form a sheet like structure. In allotrope C, each atom of element E is surrounded by four other E atoms to form a rigid structure.
(a) Name the element E.
(b) What is allotrope A.
(c) What is allotrope B?
(d) What is allotrope C?
(e) Which allotrope is used in making jewellery?
(f) Which allotrope is used in making anode of a dry cell?
What happens when methane (natural gas) burns in air? Write the chemical equation of the reaction involved.
Complete the following:
When the nuclei of two different reacting atoms are of ______ mass, then a bond so formed is called ______ covalent bond. (equal, unequal, polar, non-polar)
Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the options A, B, C and D given below:
The type of bonding in X will be ______.
What are the term defined below?
A bond formed by share pair of electrons, each bonding atom contributing one electron to the pair.
Draw the electron dot diagram and structure of nitrogen molecule.
Draw the electron dot diagram and structure of magnesium chloride.
State the type of bond formed when the combining atom has zero E.N. difference.
Taking MgCl2 as an electrovalent compound, CCl4 as a covalent compound, give four difference between electrovalent and covalent compounds
Potassium chloride is an electrovalent compound, while hydrogen chloride is a covalent compound, But, both conduct electricity in their aqueous solutions. Explain.
Name the types of Hydrocarbons.
Your body is made up of carbon.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
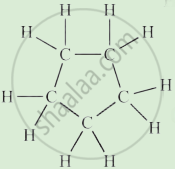 |
When ethyl alcohol and acetic acid are mixed, the resulting ester has a chemical formula ______.
The I.U.P.A.C name of CH3CH2CH = CH2 is?
In covalent compounds, the bond is formed due to ______ of electrons.
