English Medium
Academic Year: 2023-2024
Date & Time: 2nd March 2024, 10:30 am
Duration: 3h
Advertisements
General Instructions:
- This question paper comprises 39 questions. All questions are compulsory.
- This question paper is divided into five sections - A, B, C, D, and E.
- Section A: Question Nos. 1 to 20 are multiple choice questions. Each question carries 1 mark.
- Section B: Question Nos. 21 and 26 are very short answer (VSA) type questions. Each question carries 2 marks. Answer to these questions should be in the range of 30 to 50 words.
- Section C: Question Nos. 27 to 33 are short answer (SA) type questions. Each question carries 3 marks. Answer to these questions should be in the range of 50 to 80 words.
- Section D: Question Nos. 34 to 36 are long answer (LA) type questions. Each question carries 5 marks. Answer to these questions should be in the range of 80 to 120 words.
- Section E: Question Nos. 37 to 39 are 3 source-based/case-based units of assessment carrying 4 marks each with sub-parts.
- There is no overall choice. However, an internal choice has been provided in some sections. Only one of the alternatives has to be attempted in such questions.
Consider the following statements about homologous series of carbon compounds:
- All succeeding members differ by - \[\ce{CH2}\] unit.
- Melting point and boiling point increases with increasing molecular mass.
- The difference in molecular masses between two successive members is 16 u.
- \[\ce{C2H2}\] and \[\ce{C3H4}\] are NOT the successive members of alkyne series.
The correct statements are:
a and b
b and c
a and c
c and d
Chapter:
The number of shells required to write the electronic configuration of Potassium (At. No. 19).
1
2
3
4
Chapter:
Select from the following a process in which a combination reaction is involved:
Black and white photography
Burning of coal
Burning of methane
Digestion of food
Chapter:
The oxide which can react with \[\ce{HCl}\] as well as \[\ce{KOH}\] to give corresponding salt and water is:
\[\ce{CuO}\]
\[\ce{Al2O3}\]
\[\ce{Na2O}\]
\[\ce{K2O}\]
Chapter:
Which of the following is an alloy of copper and tin?
Nichrome
Brass
Constantan
Bronze
Chapter:
Tooth decay begins at the pH of ______.
5.1
5.8
6.5
8.0
Chapter:
Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by the liberation of heat. From the information given above it may be concluded that this reaction ______.
is endothermic and pH of the solution formed is more than 7
is exothermic and pH of the solution formed is 7
is endothermic and pH of the solution formed is 7
is exothermic and pH of the solution formed is more than 7
Chapter:
In human respiratory system, when a person breathes in, the position of ribs and diaphragm will ______.
lifted ribs and curve/dome shaped diaphragm.
lifted ribs and flattened diaphragm.
relaxed ribs and flattened diaphragm.
relaxed ribs and curve/dome shaped diaphragm.
Chapter:
Which of the following endocrine gland does not occur as a pair in the human body?
Adrenal
Pituitary
Testis
Ovary
Chapter: [0.06] Control and Co-ordination
Which of the following statement(s) is (are) true about human heart?
- Right atrium receives oxygenated blood from lungs through pulmonary artery.
- Left atrium transfers oxygenated blood to left ventricle which sends it to various parts of the body.
- Right atrium receives deoxygenated blood through vena cava from upper and lower body.
- Left atrium transfers oxygenated blood to aorta which sends it to different parts of the body.
a
b and d
a and d
b and d
Chapter:
Which one of the. following organism is represented by this diagram?
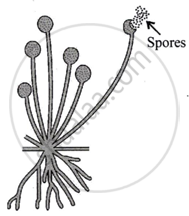
Spirogyra
Planaria
Yeast
Rhizopus
Chapter:
A cross made between two pea plants produces 50% tall and 50% short pea plants. The gene combination of the parental pea plants must be ______.
Tt and Tt
TT and Tt
Tt and tt
TT and tt
Chapter:
Strength of magnetic field produced by a current carrying solenoid does not depend upon ______.
number of turns in the solenoid
direction of the current flowing through it
radius of solenoid
material of core of the solenoid
Chapter:
SI unit of electrical resistivity is ______.
ohm per metre3
ohm per metre2
ohm. metre
ohm. metre3
Chapter:
The minimum resistance which can be made using five resistors each of resistance 10 Ω is ______.
`1/50 Omega`
`1/5 Omega`
2 Ω
1 Ω
Chapter:
Consider the following statements in the context of human eye:
- The diameter of the eye ball is about 2.3 cm.
- Iris is a dark muscular diaphragm that controls the size of the pupil.
- Most of the refraction for the light rays entering the eye occurs at the crystalline lens.
- While focusing on the objects at different distances, the distance between the crystalline lens and the retina is adjusted by ciliary muscles.
The correct statements are:
a and b
a, b and c
b, c and d
a, c and d
Chapter:
Assertion (A): The deflection of a compass needle placed near a current carrying wire decreases when the magnitude of an electric current in the wire is increased.
Reason (R): Strength of the magnetic field at a point due to a current carrying conductor increases on increasing the current in the conductor.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter:
Assertion (A): Human female has a perfect pair of sex chromosome.
Reason (R): Sex chromosome contributed by the human male in the zygote decides the sex of a child.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter:
Assertion (A): Myopic eye cannot see distant objects distinctly.
Reason (R): For the correction of myopia converging lenses of appropriate power are prescribed by eye surgeons.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter:
Assertion (A): Metals in the middle of activity series are found in nature as sulphides or carbonates.
Reason (R): The sulphide ores are calcinated whereas carbonate ores are roasted to extract metals from them.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter:
Write an equation to show thermal decomposition of ferrous sulphate crystals.
Chapter:
Why is it necessary for the equation to be balanced?
Chapter:
| Two test tubes A and B are taken, each containing one ml of starch solution. Add 1 ml of saliva to test tube 'A' only and leave both the test tubes undisturbed for a few minutes. Now add a few drops of dilute iodine solution to both the test tubes. |
- Which one of the two test tubes shows change in colour? Write the changed colour observed in this test tube.
- What can we conclude from this activity?
Chapter:
Name two types of germ cells present in human beings. List two structural differences between the two.
Chapter:
Advertisements
Chapter: [0.09] Light - Reflection and Refraction
A ray of light enters from, vacuum to glass of absolute refractive index 1.5. Find the speed of light in glass. The speed of light in vacuum is 3 × 108 m/s.
Chapter:
Use Ohm's law to determine the potential difference across the 3 Ω resistor in the circuit shown in the following diagram when key is closed:

Chapter:
Name the term used for the materials which cannot be broken down by biological processes. Give two ways by which they harm various components of an ecosystem.
Chapter:
Give reasons for the following:
Alveoli in lungs are richly supplied with blood capillaries.
Chapter:
Give reasons for the following:
Respiratory pigment in the blood takes up oxygen and not carbon dioxide.
Chapter:
Give reasons for the following:
During anaerobic respiration, a 3-carbon molecule is formed as an end product instead of CO2 in human beings.
Chapter:
Name the movements that occur all along the gut in human digestive system. How do they help in digestion?
Chapter:
In angiosperms why fertilisation cannot take place in flowers if pollination does not take place? Where is zygote located in a flower after fertilisation? What does it develop into?
Chapter:
Write the names of those parts of a flower which serve the same function as the following do in animals:
Chapter:
State any two observations when an electric current is passed through acidulated water, in a container having each electrode covered by test tubes filled with water.
Chapter:
Write the ratio of the mass of the gas collected at the cathode to the mass of the gas collected at the anode.
Chapter:
Draw a labelled diagram to show electrolytic refining of copper. State what happens when electric current is passed through the electrolyte taken in this case.
Chapter:
An object is placed in front of a concave mirror of focal length 12 cm. If distance of the object from the pole of the mirror is 8 cm, then use mirror formula to determine the position of the image formed. Draw a labelled ray diagram to justify your answer in this case.
Chapter:
The image of an object formed by a mirror is real, inverted and is of magnification −1. If the image is at a distance of 30 cm from the mirror, where is the object placed? Give reason to justify your answer.
Chapter:
Where would the image be if the object is moved 15 cm towards the mirror? Draw ray diagram for the new position of the object to justify your answer.
Chapter:
State Fleming's left hand rule. Apply this rule to determine the direction of force experienced by a straight current carrying conductor AB placed in a uniform magnetic field as shown.
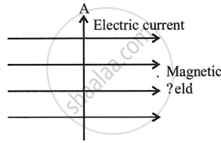
Chapter:
Advertisements
What will happen to an electron which enters in the same field in the same direction in which the current is flowing in the conductor AB? Give reason to justify your answer.

Chapter:
Use of pesticides to protect our crops affect organisms at various trophic levels especially human beings. Name the phenomenon involved and explain how does it happen.
Chapter:
Upper half of a convex lens is covered with a black paper. Draw a ray diagram to show the formation of image of an object placed at a distance of 2F from such a lens. Mention the position and nature of the image formed. State the observable difference in the image obtained if the lens is uncovered. Give reason to justify your answer.
Chapter:
An object is placed at a distance of 30 cm from the optical centre of a concave lens of focal length 15 cm. Use lens formula to determine the distance of the image from the optical centre of the lens.
Chapter:
State the reason why carbon can neither form \[\ce{C^4+}\] cations nor \[\ce{C^4−}\] anions but forms covalent compound.
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
What is meant by homologous series of carbon compounds?
Chapter: [0.04] Carbon and its Compounds
Write the molecular formula of two consecutive members of homologous series of aldehydes. State which part of these compounds determines their
- physical and
- chemical properties
Chapter: [0.04] Carbon and its Compounds
Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in a molecule of cyclohexane?
Chapter: [0.04] Carbon and its Compounds
- Name a commercially important carbon compound having functional group - OH and write its molecular formula.
- Write chemical equation to show its reaction with
- Sodium metal
- Excess conc. sulphuric acid
- Ethanoic acid in the presence of an acid catalyst
- Acidified potassium dichromate
Also write the name of the product formed in each case.
Chapter:
Distinguish between hormonal co-ordination in plants and animals.
Chapter:
Which part of the brain is responsible for intelligence?
Chapter:
Which part of the brain is responsible for riding a bicycle?
Chapter:
Which part of the brain is responsible for vomiting?
Chapter:
Which part of the brain is responsible for controlling hunger?
Chapter:
How is human brain protected from mechanical injuries and shocks?
Chapter:
Give an example of a plant hormone which inhibits growth.
Chapter:
Give an example of a plant hormone which promotes cell division.
Chapter:
Explain directional movement of a tendril in pea plant in response to touch. Name the hormone responsible for this movement.
Chapter:
|
When electric current flows in a purely resistive circuit electrical energy gets fully converted into heat energy. The amount of heat produced (H) in the circuit is found to be directly proportional to
In other words H = I2Rt. Electrical devices such an electric fuse, electric heater, electric iron etc. are all based on this effect called heating effect of electric current. |
- List two properties of heating elements. 1
- List two properties of electric fuse. 1
- Name the principle on which an electric fuse works. Explain how a fuse wire is capable of saving electrical appliances from getting damaged due to accidently produced high currents. 2
OR
The power of an electric heater is 1100 W. If the potential difference between the two terminals of the heater is 220 V, find the current flowing in the circuit. What will happen to an electric fuse of rating 5 A connected in this circuit? 2
Chapter:
| Salts play a very important role in our daily life. Sodium chloride which is known as common salt is used almost in every kitchen. Baking soda is also a salt used in faster cooking as well as in baking industry. The family of salts is classified on the basis of cations and anions present in them. |
- Identify the acid and base from which Sodium chloride is formed. 1
- Find the cation and the anion present in Calcium sulphate. 1
- "Sodium chloride and washing soda both belong to the same family of salts." Justify this statement. 2
OR
Define the term pH scale. Name the salt obtained by the reaction of Potassium hydroxide and Sulphuric acid and give the pH value of its aqueous solution. 2
Chapter:
|
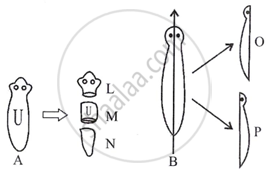
Asexual reproduction involves a single parent to produce offsprings without the formation of gametes. It occurs by the following ways: Fission, Budding, Fragmentation, Spore formation and Regeneration. In one of the methods like regeneration, Planaria A is cut horizontally into three pieces L, M and N and Planaria B is cut vertically into two equal halves - O and P.
|
- Which of the cut pieces of the two Planaria could regenerate to form a complete organism? 1
- Give an example of another organism that follows the same mode of reproduction as Planaria. 1
- What is the meaning of 'development' in regeneration? 2
OR
Differentiate between regeneration and fragmentation. 2
Chapter:
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2023 - 2024
Previous year Question paper for CBSE Class 10 Science-2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.