Advertisements
Advertisements
Question
Write the molecular formula and structure of benzene.
Solution
The molecular formula of benzene is C6H6.
Its structural formula is :
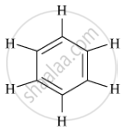
APPEARS IN
RELATED QUESTIONS
What is meant by isomers?
Draw the structures for Ethanoic acid.
Give reason why the carbon compounds do not conduct electricity in the molten state.
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
benzene
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if A is a cyclic compound
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if B is an open chain compound
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains only single bonds?
The solid element A exhibits the property of catenation. It is also present in the form of a gas B in the air which is utilised by plants in photosynthesis. An allotrope C of this element is used in glass cutters.
(a) What is element A?
(b) What is the gas B?
(c) Name the allotrope C.
(d) State another use of allotrope C (other than in glass cutters).
(e) Name another allotrope of element A which exists as spherical molecules.
(f) Name a yet another allotrope of element A which conduct electricity.
Which of the following are correct structural isomers of butane?
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.....}\backslash\phantom{..}|\\
\phantom{.....}\ce{H}\phantom{.......}\ce{C - H}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{....}\phantom{....}|\\
\ce{H}\phantom{........}\ce{H}\\
|\\
\ce{H - C - H}\\
|\\\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}
\end{array}\]
The number of isomers of pentane is ______.
