Advertisements
Advertisements
Question
State any two uses of graphite.
Solution
Uses of graphite:
(1) As graphite is a good conductor of electricity, it is used in making carbon electrodes in dry cells.
(2) Graphite is used in making pencil leads and black paints.
APPEARS IN
RELATED QUESTIONS
Name the type of bonds formed in ionic compounds and in the compounds formed by carbon.
What type of bonds are present in CO2 molecule? Draw their electron-dot structures.
Fill in the blank in the following sentence:
The number of single covalent bonds in C2H2 molecule are ...........
Explain why, ionic compounds conduct electricity in solution whereas covalent compounds do not conduct electricity.
Draw the electron-dot structure of N2 and state the type of bonding.
Write two points of difference in the structures of diamond and graphite.
Explain why, diamond can be used in rock drilling equipment but graphite cannot.
What is graphite?
what substance is graphite made?
Give the formulae of the chlorides of the elements X and Y having atomic numbers of 3 and 6 respectively. Will the properties of the two chlorides be similar or different? Explain your answer.
What happens when methane (natural gas) burns in air? Write the chemical equation of the reaction involved.
Taking hydrogen chloride and methane as examples, distinguish between a polar covalent bond and a non polar covalent bond.
Explain the following:
Polar covalent compounds are good conductors of electricity.
Elements Q and S react together to form an ionic compound. Under normal conditions, which physical state will the compound QS exist in?
Draw the electron dot structure of covalent compound methane (non polar) and HCL (polar) and give two difference between them.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
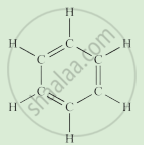 |
Show the covalent bond formation in nitrogen molecule.
The number of single and double bonds present in a molecule of benzene (C6H6) respectively, are ______.
