Advertisements
Advertisements
Question
Draw the electron dot structure of covalent compound methane (non polar) and HCL (polar) and give two difference between them.
Solution
| Methane (non polar) | HCL (polar) |
| Covalent compounds are non polar when shared pairs of electron(s) are equally distributed between the two atoms. | Covalent compounds are said to be polar when the shared pair of electrons are not at equal distance between the two atoms. |
| No charge separation takes place. The covalent molecule is symmetrical and electrically neutral. | Charge separation takes place. The atom which attracts electrons more strongly develops a slight negative charge while the other creates a little positive charge. |
| \[\begin{array}{cc} \ce{H}\\ \ce{H:\underset{..}{\overset{..}{C}} :H}\\ \ce{H} \end{array}\] |
\[\ce{H:\underset{..}{\overset{..}{Cl}}}\] |
APPEARS IN
RELATED QUESTIONS
Name a carbon containing molecule which has two double bonds.
Explain why, ionic compounds conduct electricity in solution whereas covalent compounds do not conduct electricity.
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of oxygen, O2, is formed from two oxygen atoms. What name is given to this type of bonding? (At. No. of oxygen = 8)
What is graphite?
Buckminsterfullerene is an allotropic form of the element:
(a) phoshorus
(b) fluorine
(c) carbon
(d) sulphur
The molecules having triple bond in them are:
(a) oxygen and ethyne
(b) carbon dioxide and ammonia
(c) methane and ethene
(d) nitrogen and ethyne
What is the difference between ionic compounds and covalent compounds?
Explain the following:
Water is a polar covalent molecule.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
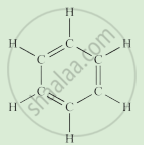 |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
