Advertisements
Advertisements
Question
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of oxygen, O2, is formed from two oxygen atoms. What name is given to this type of bonding? (At. No. of oxygen = 8)
Solution
The atomic number of oxygen is 8. Its electronic configuration is 2, 6. Hence it has six electrons in its outermost shell.
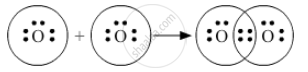
In an oxygen molecule, one oxygen atom shares its two electrons with another oxygen atom. Hence this type of bonding is called covalent bonding.
APPEARS IN
RELATED QUESTIONS
List three characteristic properties of covalent compounds.
Name the type of bonds formed in ionic compounds and in the compounds formed by carbon.
Why are most carbon compounds poor conductors of electricity?
Name the black substance of pencil. Will the current flow through the electrical circuit when we use the sharpened ends of the pencil to complete the circuit?
What type of bonds are present in water molecule? Draw the electron-dot structure of water (H2O).
What type of bonds are present in methane (CH4) and sodium chloride (NaCl)?
Give one example of a molecule containing a double covalent bond
Draw the electron-dot structure of H2O compound and state the type of bonding.
The solution of one of the following compounds will not conduct electricity. This compounds is:
(a) NaCl
(b) CCl4
(c) MgCl2
(d) CaCl2
One of the following contains a double bond as well as single bonds. This is:
(a) CO2
(b) O2
(c) C2H4
(d) C2H2
Define a coordinate bond and give the conditions for its formation.
Explain the following term with example.
Hetero atom in a carbon compound
What do you understand by lone pair and shared pair?
What is the term defined below?
A bond formed by a shared pair of electrons, each bonding atom contributing one electron to the pair.
Draw the electron dot diagram and structure of nitrogen molecule.
Element A has 2 electrons in its M shell. Element B has atomic number 7.
Write equations to show how A and B form ions.
Potassium (Atomic No. 19) and chlorine (Atomic No. 17) react to form a compound. On the basis of electronic concept, explain
(i) oxidation
(ii) reduction
(iii)oxidising agent
(iv)reducing agent
Identify the incorrect statement and correct them.
- Like covalent compounds, coordinate compounds also contain charged particles (ions). So they are good conductors of electricity.
- Ionic bond is a weak bond when compared to Hydrogen bond.
- Ionic or electrovalent bonds are formed by mutual sharing of electrons between atoms.
- Loss of electrons is called Oxidation and gain of electron is called Reduction.
- The electrons which are not involved in bonding are called valence electrons.
Molecular reactions are ______ in the covalent compound.
What is a covalent bond?
