Advertisements
Advertisements
प्रश्न
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of oxygen, O2, is formed from two oxygen atoms. What name is given to this type of bonding? (At. No. of oxygen = 8)
उत्तर
The atomic number of oxygen is 8. Its electronic configuration is 2, 6. Hence it has six electrons in its outermost shell.
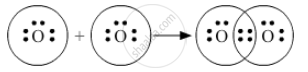
In an oxygen molecule, one oxygen atom shares its two electrons with another oxygen atom. Hence this type of bonding is called covalent bonding.
APPEARS IN
संबंधित प्रश्न
Write the number of covalent bonds in the molecule of butane, C4H10.
Why covalent compounds are different from ionic compounds?
Ethane, with the molecular formula C2H6 has ______.
Write any three features and give two examples of covalent compounds
State whether the following statement is true or false:
Diamond and graphite are the covalent compounds of carbon element (C)
Name a carbon containing molecule which has two double bonds.
Explain why, ionic compounds conduct electricity in solution whereas covalent compounds do not conduct electricity.
What is graphite?
Describe the structure of graphite with the help of a labelled diagram.
A covalent molecule having a double bond between its atoms is:
(a) Hydrogen
(b) Oxygen
(c) water
(d) ammonia
Give the characteristic properties of covalent compounds.
Give examples for the following:
Two gaseous polar compounds.
What do you understand by lone pair and shared pair?
Explain the following:
Covalent compounds have low melting and boiling point.
Name two compounds that are covalent when taken pure but produce ions when dissolved in water.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
The I.U.P.A.C name of CH3CH2CH = CH2 is?
Which of the following statements are correct for carbon compounds?
- Most carbon compounds are good conductors of electricity.
- Most carbon compounds are poor conductors of electricity.
- Force of attraction between molecules of carbon compounds is not very strong.
- Force of attraction between molecules of carbon compounds is very strong.
A molecule of ammonia (NH3) has
