Advertisements
Advertisements
प्रश्न
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of oxygen, O2, is formed from two oxygen atoms. What name is given to this type of bonding? (At. No. of oxygen = 8)
उत्तर
The atomic number of oxygen is 8. Its electronic configuration is 2, 6. Hence it has six electrons in its outermost shell.
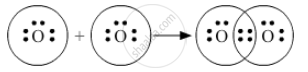
In an oxygen molecule, one oxygen atom shares its two electrons with another oxygen atom. Hence this type of bonding is called covalent bonding.
APPEARS IN
संबंधित प्रश्न
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – the eight atoms of sulphur are joined together in the form of a ring.)
Explain the bonding in methane molecule using electron dot structure.
What type of chemical bond is present in chlorine molecule? Explain your answer.
Compare the properties of ionic compounds and covalent compounds.
State any two uses of graphite.
The organic compound prepared by Wohler from an inorganic compound called ammonium cyanate was:
(a) glucose
(b) urea
(c) uric acid
(d) vinegar
The number of carbon atoms joined in a spherical molecule of buckminsterfullerene is:
(a) fifty
(b) sixty
(c) seventy
(d) ninety
What happens when methane (natural gas) burns in air? Write the chemical equation of the reaction involved.
What is the difference between ionic compounds and polar covalent compounds?
Complete the following:
When the nuclei of two different reacting atoms are of ______ mass, then a bond so formed is called ______ covalent bond. (equal, unequal, polar, non-polar)
Explain the following term with example.
Alkane
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
X is likely to have a :
Acids dissolve in water to produce positively charged ions. Draw the structure of these positive ions.
Draw the electron dot diagram and structure of magnesium chloride.
Define the functional group and complete the following table.
| S.I. No. | Functional Group | Compound | Formula |
| (1) | ______________ | _______________ |
C2H5OH |
| (2) | _______________ | _______________ | CH3CHO |
List two differences between the properties exhibited by covalent compounds and ionic compounds.
Discuss in brief about the properties of coordinate covalent compounds.
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
Show the covalent bond formation in nitrogen molecule.
