Advertisements
Advertisements
Question
What do you understand by lone pair and shared pair?
Solution
Lone pair: A pair of electrons which is not shared with any other atom is known as the lone pair of electrons.
For example in NH3, Nitrogen has a lone pair of electrons which is not shared with any hydrogen atom.
Shared pair: A pair of electrons which is shared with other atoms to form a bond is known as shared pair of electrons.
For example in HCl the pair of electrons responsible for bond formation between H and Cl is called shared pair.
APPEARS IN
RELATED QUESTIONS
Give the formula of the compound that would be formed by the combination of the following pair of elements:
Al and Cl2
The solution of one of the following compounds will not conduct electricity. This compounds is:
(a) NaCl
(b) CCl4
(c) MgCl2
(d) CaCl2
Which of the following cannot exhibit isomerism?
(a) C4H10
(b) C5H12
(c) C3H8
(d) C6H14
What are the conditions necessary for the formation of covalent molecules?
Explain the following:
polar covalent compounds electricity.
The following structural formula belongs to which carbon compound?
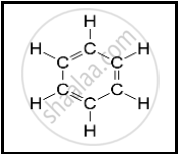
Give examples for the following:
Two gaseous polar compounds.
Elements Q and S react together to form an ionic compound. Under normal conditions, which physical state will the compound QS exist in?
Covalent bonds can be single, double or triple covalent bonds. How many electrons are shared in each? Give an example of each type.
Taking MgCl2 as an electrovalent compound, CCl4 as a covalent compound, give four difference between electrovalent and covalent compounds
