Advertisements
Advertisements
Question
Taking MgCl2 as an electrovalent compound, CCl4 as a covalent compound, give four difference between electrovalent and covalent compounds
Solution
|
MgCl2 - Electrovalent compound |
CCl4 - Covalent compound |
|
They are hard crystalline solids consisting of ions. |
These are gases or liquids or soft solids. |
|
They have high melting and boiling points. |
They have low melting and boiling points. |
|
They conduct electricity in the fused or aqueous state. |
They do not conduct electricity in the solid, molten or aqueous state. |
|
These are soluble in inorganic solvents but insoluble in organic solvents. |
These are insoluble in water but dissolve in organic solvents. |
APPEARS IN
RELATED QUESTIONS
State the reason why carbon can neither form \[\ce{C^4+}\] cations nor \[\ce{C^4−}\] anions but forms covalent compound.
An element L consists of molecules.
What type of bonding is present in the particles that make up L?
Why are most carbon compounds poor conductors of electricity?
What type of bonds are present in hydrogen chloride and oxygen?
What is graphite?
State the type of bonding in the following molecule.
Hydroxyl ion
Give two example in following case:
Solid covalent compounds
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
The type of bonding in X will be
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
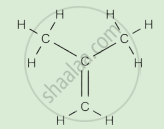 |
Explain dipole (polar) molecule by taking hydrogen chloride as an example.
