Advertisements
Advertisements
Question
Give the name and structural formula of one member each of the following:
alkyne
Solution
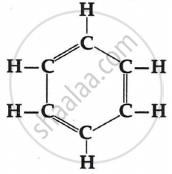
Ethyne is an alkyne with the molecular formula C2H2.
Structural formula is
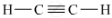
APPEARS IN
RELATED QUESTIONS
List two reasons for carbon forming a large number of compounds. Name the type of bonding found in most of its compounds. Why does carbon form compounds mainly by this kind of bonding?
Draw the structures for Ethanoic acid.
Draw the structures for Bromopentane*
Write the molecular formula and structure of benzene.
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if B is an open chain compound
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which formula represent cyclohexane as well as hexene?
The molecular formulae of some organic compounds are given below. Which of these compounds contains an aldehyde group?
(a) C3H8O
(b) C3H6O2
(c) C3H6O
(d) C3H7Cl
Match the reactions given in Column (A) with the names given in column (B).
| Column (A) | Column (B) | ||
(a) |
`"CH"_3"OH" + "CH"_3"COOH"overset("H"^+)(->) "CH"_3"COOCH"_3 + "H"_2"O"` | (i) | Addition reaction |
| (b) | `"CH"_3 = "CH"_2 + "H"_2 overset("Ni")(->)"CH"_3 - "CH"_3` | (ii) | Substitution reaction |
| (c) | `"CH"_4 + "Cl"_2overset("Sunlight")(->)"CH"_3"Cl" + "HCl"` | (iii) | Neutralisation reaction |
| (d) | `"CH"_3"COOH" + "NaOH" -> "CH"_3"COONa" + "H"_2"O"` | (iv) | Esterification reaction |
Recognise the carbon chain type of the following:
\[\begin{array}{cc}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\\ce{H - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\\\ce{H}\phantom{...}\ce{}\phantom{.....}\ce{H}\\
\ce{H - C - H}\\
|\\
\ce{H}
\end{array}\]
Name the following: