Advertisements
Advertisements
Question
Write the electron-dot structures for ethyne.
Solution
Ethyne:
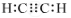
shaalaa.com
Is there an error in this question or solution?
APPEARS IN
RELATED QUESTIONS
How many structural isomers can you draw for pentane?
Draw the electron dot structures for F2.
Fill in the following blank with suitable word:
Ethyne has ......... carbon-hydrogen single bonds.
Which of the following hydrocarbons is unsaturated?
C3H4; C2H6
Name a cyclic unsaturated carbon compound.
Structural formula of ethyne is
Identify the unsaturated compounds from the following
- Propane
- Propene
- Propyne
- Chloropropane
What is the role of metal or reagents written on arrows in the given chemical reactions?
- \[\begin{array}{cc}
\ce{CH3}\phantom{.....}\ce{CH3}\phantom{.........}\ce{CH3}\phantom{..}\ce{CH3}\phantom{..........}\\
\phantom{...}\backslash\phantom{......}/\phantom{................}|\phantom{.....}|\phantom{............}\\
\ce{C} = \ce{C} + \ce{H2} \overset{\ce{Ni}}{\rightarrow} \ce{CH3 - C - C - CH3}\\
\phantom{.}/\phantom{........}\backslash\phantom{..............}|\phantom{....}|\phantom{.........}\\
\ce{CH3}\phantom{....}\ce{CH3}\phantom{............}\ce{H}\phantom{...}\ce{H}\phantom{........}
\end{array}\] - \[\ce{CH3COOH + CH3CH2OH \overset{\ce{Conc. H2SO4}}{\rightarrow}CH3COOC2H5 + H2O}\]
- \[\ce{CH3CH2OH}\ce{->[Alk.KMnO4][Heat]CH3COOH}\]
The formulae of four organic compounds are shown below. Choose the correct option
| A | B | C | D |
| \[\begin{array}{cc} \phantom{.}\ce{H}\phantom{......}\ce{H}\\ \phantom{.}\backslash\phantom{.....}/\\ \ce{C = C}\\ /\phantom{.....}\backslash\\ \ce{H}\phantom{......}\ce{H} \end{array}\] |
\[\begin{array}{cc} \phantom{........}\ce{H}\phantom{.....}\ce{O}\\ \phantom{.......}|\phantom{....}//\\ \ce{H - C - C}\\ \phantom{......}|\phantom{.....}\backslash\\ \phantom{...........}\ce{H}\phantom{.....}\ce{O - H} \end{array}\] |
\[\begin{array}{cc}
|
\[\begin{array}{cc} \ce{H}\phantom{...}\ce{H}\phantom{....}\\ |\phantom{....}|\phantom{....}\\ \ce{H - C - C - O - H}\\ |\phantom{....}|\phantom{.....}\\ \ce{H}\phantom{....}\ce{H}\phantom{.....} \end{array}\] |
- Write the name and draw the structure of a saturated hydrocarbon with four carbon atoms.
- Write the number of single covalent bonds present in this compound.
