Advertisements
Advertisements
Question
The structural formula of an ester is :

Write the formula of the acid and the alcohol from which it is formed.
Solution
he structural formula of the ester corresponds to the compound ethylethanoate (CH3COOC2H5). Esters are sweet smelling substances which are formed when an alcohol reacts with carboxylic acid in the presence of concentrated sulphuric acid.
(a) The formula of the ethanoic acid is CH3COOH.
(b) The formula of the alcohol (ethanol) is CH3-CH2-OH.
The chemical equation showing the reactants and the products is as follows:
`CH_3 COOH + CH_3 CH_2 OH` 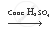 `CH_3 COOCH_2 CH_3 + H_2 O`
`CH_3 COOCH_2 CH_3 + H_2 O`
APPEARS IN
RELATED QUESTIONS
How is ethanoic acid obtained from ethanol? Write down the chemical equation of the reaction involved.
Which of the following molecular formula corresponds to ethylbutanoate ester?
(a) C5H10O2
(b) C6H12O2
(c) C7H14O2
(d) C8H16O2
A neutral organic compound is warmed with some ethanoic acid and a little of conc. H2SO4. Vapours having sweet smell (fruity smell) are evolved. What type of functional group is present in this organic compound?
Which one of the following are the correct observations about acetic acid?
(A) It turns blue litmus red and smells like vinegar
(B) It turns blue litmus red and smells like burning sulphur
(C) It turns res litmus blue and smells like vinegar
(D) It turns red litmus blue and has a fruity smell
Identify the term or substance based on the descriptions given below:
Ice like crystals formed on cooling an organic acid sufficiently.
Write the characteristics of ethanoic acid.
A student while observing the properties of acetic acid would report that this smells like ______.
Ethanoic acid reacts with the basic salt, sodium carbonate, to form a salt, named ______, water and carbon dioxide gas.
A spatula full of sodium carbonate is taken in a test tube and 2 mL of dilute ethanoic acid is added to it.
Suggest a method of testing the gas liberated in the reaction.
Name the oxidising agent used in the conversion of ethanol to ethanoic acid.
