Advertisements
Advertisements
प्रश्न
The structural formula of an ester is :

Write the formula of the acid and the alcohol from which it is formed.
उत्तर
he structural formula of the ester corresponds to the compound ethylethanoate (CH3COOC2H5). Esters are sweet smelling substances which are formed when an alcohol reacts with carboxylic acid in the presence of concentrated sulphuric acid.
(a) The formula of the ethanoic acid is CH3COOH.
(b) The formula of the alcohol (ethanol) is CH3-CH2-OH.
The chemical equation showing the reactants and the products is as follows:
`CH_3 COOH + CH_3 CH_2 OH` 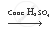 `CH_3 COOCH_2 CH_3 + H_2 O`
`CH_3 COOCH_2 CH_3 + H_2 O`
APPEARS IN
संबंधित प्रश्न
When zinc powder is added to acetic acid ______________
(a) the mixture becomes warm
(b) a gas is evolved
(c) the colour of the mixture becomes yellow
(d) a solid settles at the bottom
Write the IUPAC names, common names and formulae of the first two members of the homologous series of carboxylic acids.
Draw the structure for the following compound.
Ethanoic acid
Three organic compounds A, B and C have the following molecular formulae: C4H10O
Which compound contains a carboxyl group? Write its name and structural formula.
What happens when ethanoic acid reacts with sodium carbonate? Write chemical equation of the reaction involved.
What is an oxidising agent?
A neutral organic compound is warmed with some ethanoic acid and a little of conc. H2SO4. Vapours having sweet smell (fruity smell) are evolved. What type of functional group is present in this organic compound?
Vinegar is a solution of ______.
Reshu by mistake forgot to label the two test tubes containing Ethanol and Ethanoic acid. Suggest an experiment to identify the substances correctly? Illustrate the reactions with the help of chemical equations.
Write the chemical equation for the following:
Esterification Reaction
