Advertisements
Advertisements
प्रश्न
Reshu by mistake forgot to label the two test tubes containing Ethanol and Ethanoic acid. Suggest an experiment to identify the substances correctly? Illustrate the reactions with the help of chemical equations.
उत्तर
By reaction with sodium carbonate/ bi carbonate 1M with the samples, ethanol will not react whereas ethanoic acid gives brisk effervescence.
\[\ce{2CH3COOH + Na2 CO3 -> 2CH3COONa +H2O + CO2}\]
OR
\[\ce{CH3COOH + NaHCO3 -> CH3COONa + H2O + CO2}\]
APPEARS IN
संबंधित प्रश्न
Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 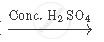
Choose those compounds from the following which can turn blue litmus solution red:
HCHO, CH3COOH, CH3OH, C2H5OH, HCOOH, CH3CHO
Give reasons for your choice.
Name the reducing agent used to convert acetic acid into ethanol?
On adding acetic acid to sodium hydrogen carbonate in a test tube, a student observes
(A) no reaction
(B) a colourless gas with pungent smell
(C) bubbles of a colourless and odourless gas
(D) a strong smell of vinegar
Why is Soda lime used not only NaOH?
Choose the correct word/phrase from the options given below to complete the following sentence:
When acetaldehyde is oxidized with acidified potassium dichromate, it forms ______.
With a labelled diagram describe in brief an activity to show the formation of ester.
Write the characteristics of ethanoic acid.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
