Advertisements
Advertisements
प्रश्न
The structural formula of an ester is :

Write the formula of the acid and the alcohol from which it is formed.
उत्तर
he structural formula of the ester corresponds to the compound ethylethanoate (CH3COOC2H5). Esters are sweet smelling substances which are formed when an alcohol reacts with carboxylic acid in the presence of concentrated sulphuric acid.
(a) The formula of the ethanoic acid is CH3COOH.
(b) The formula of the alcohol (ethanol) is CH3-CH2-OH.
The chemical equation showing the reactants and the products is as follows:
`CH_3 COOH + CH_3 CH_2 OH` 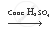 `CH_3 COOCH_2 CH_3 + H_2 O`
`CH_3 COOCH_2 CH_3 + H_2 O`
APPEARS IN
संबंधित प्रश्न
Complete the following chemical equations :CH3COOH+NaOH→
Draw the structure for the following compound.
Ethanoic acid
What is glacial acetic acid ?
Name the reducing agent used to convert acetic acid into ethanol?
What do you observe when acetic acid is added to ethyl alcohol in the presence of sulphuric acid?
Which of the following observations is true about dilute solution of acetic acid?
(A) It smells like vinegar and turns red litmus blue
(B) It smells like onion and turns blue litmus red
(C) It smells like orange and turns red litmus blue
(D) It smells like vinegar and turns blue litmus red
Choose the correct option from given alternative:
When sodium hydrogen carbonate is added to ethanoic acid a gas evolves. Consider the following statements about the gas evolved?
(A) It turns lime water milky.
(B) It is evolved with brisk effervescence.
(C) It has a smell of burning sulfur.
(D) It is also a by-product of respiration.
A student while observing the properties of acetic acid would report that this smells like ______.
Which of these is not an organic acid?
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
