Advertisements
Advertisements
प्रश्न
What is an oxidising agent?
Explain the term Oxidising agent with example.
उत्तर १
An oxidising agent is a reactant which readily transfers oxygen atoms to another substance by reducing itself in the process.
उत्तर २
The substances which can give oxygen to other substances are called oxidant or oxidizing agents.
E.g. Potassium permanganate (KMnO4) and potassium dichromate (K2Cr2O7) in acidic or alkaline medium is commonly used oxidants.
संबंधित प्रश्न
While studying saponification reaction, a student measures the temperature of the reaction mixture and also finds its nature using blue/red litmus paper. On the basis of his observations the correct conclusion would be
(A) the reaction is exothermic and the reaction mixture is acidic.
(B) the reaction is endothermic and the reaction mixture is acidic.
(C) the reaction is endothermic and the reaction mixture is basic.
(D) the reaction is exothermic and the reaction mixture is basic.
A student puts a drop of reaction mixture of a saponification reaction first a blue litmus paper and then on a red litmus paper. He may observe that:
(a) There is no change in the blue litmus paper and the red litmus paper turns white.
(b) There is no change in the red litmus paper and the blue litmus paper turns red.
(c) There is no change in the blue litmus paper and the red litmus paper turns blue.
(d) No change in colour is observed in both the litmus papers
While studying saponification reaction for the preparation of soap, a teacher suggested to a student to add a small quantity of common salt to the reaction mixture. The function of common salt in this reaction is to
(A) reduce the alkalinity of the soap
(B) reduce the acidity of the soap
(C) enhance the cleansing capacity of soap
(D) favour precipitation of soap
Complete the following chemical equations:
C2H5OH+CH3COOH`("conc."H_2SO_4)/`>
Identify the term or substance based on the descriptions given below
Ice like crystals formed on cooling an organic acid sufficiently.
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 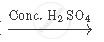
Name two oxidising agents which can oxidise ethanol to ethanoic acid.
How is ethanoic acid obtained from ethanol? Write down the chemical equation of the reaction involved.
Give any two uses of ethanoic acid.
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is a constituent of vinegar?(e) Which compound is a constituent of vinegar?
Write the IUPAC name of the following:
\[\begin{array}{cc}
\ce{CH3}\\
|\\
\ce{CH3 -C -CH3}\\
|\\
\ce{CH3}
\end{array}\]
Name the product formed and give an appropriate chemical equation for the following:
Ethanol oxidised by acidified potassium dichromate?
Which of the following observations is true about dilute solution of acetic acid?
(A) It smells like vinegar and turns red litmus blue
(B) It smells like onion and turns blue litmus red
(C) It smells like orange and turns red litmus blue
(D) It smells like vinegar and turns blue litmus red
Name the following:
A toxic alcohol
Write the important uses of acetic acid.
What is thr boilng point of acetic acid?
Fill in the blank with appropriate word/words.
Esterification is the reaction between carboxylic acid and ______ in presence of ____
State how the following conversions can be carried out:
Ethene to Ethyl alcohol
Give balanced chemical equations for the following conversion:
Calcium carbide to ethyne
A compound X when treated with an organic acid Y (having vinegar-like smell) in the presence of the acid Z, forms a compound P which has a fruity smell.
- Identify X, Y, and Z
- Write the structural formula of X and Y.
- What type of compound P is?
- Name the above reaction.
- If compound X and Y both have 2 carbon atoms. Write the reaction.
Ethanoic acid _________.
Give the balanced chemical equation of the following reaction:
Evolution of carbon dioxide by the action of ethanoic acid with NaHCO3.
Mineral acids are stronger acids than carboxylic acids because
- mineral acids are completely ionised
- carboxylic acids are completely ionised
- mineral acids are partially ionised
- carboxylic acids are partially ionised
Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
Shristi heated Ethanol with a compound A in presence of a few drops of concentrated sulphuric acid and observed a sweet smelling compound B is formed. When B is treated with sodium hydroxide it gives back Ethanol and a compound C.
- Identify A and C
- Give one use each of compounds A and B.
- Write the chemical reactions involved and name the reactions.
Raina while doing certain reactions observed that heating of substance ‘X’ with a vinegar-like smell with a substance ‘Y’ (which is used as an industrial solvent.) in the presence of conc. Sulphuric acid in a water bath gives a sweet-smelling liquid ‘Z’ having molecular formula C4H8O2. When heated with caustic soda (NaOH), ‘Z’ gives back the sodium salt and the compound ‘Y’.
Identify ‘X’, ‘Y’, and ‘Z’. Illustrate the changes with the help of suitable chemical equations.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
Give the balanced chemical equation of the following reaction:
Combustion of ethanol.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
