Advertisements
Advertisements
प्रश्न
How is ethanoic acid obtained from ethanol? Write down the chemical equation of the reaction involved.
उत्तर
When ethanol (ethyl alcohol) is heated in the presence of alkaline potassium permanganate solution or acidified potassium dichromate solution, it gets oxidised to ethanoic acid. This reaction is known as oxidation reaction.
The chemical equation for the above reaction is:
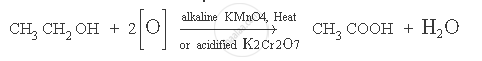
APPEARS IN
संबंधित प्रश्न
A student adds a few drops of ethanoic acid to test tubes X, Y and Z containing aqueous solutions of sodium chloride, sodium hydroxide and sodium carbonate, respectively. If he now brings a burning splinter near the mouth of the test tubes immediately after adding ethanoic acid in each one of them, in which of the test tube or test tubes the flame will be extinguished?
(A) X and Y
(B) Y and Z
(C) X and Z
(D) only Z
Complete the following chemical equations: CH3COOH+NaHCO3→
Write the formulae of methanoic acid.
State any two uses of esters.
If you are asked to report your observations about the following two properties of acetic acid, what would you report?
(i) Odour
(ii) Effect on litmus
Which one of the following are the correct observations about acetic acid?
(A) It turns blue litmus red and smells like vinegar
(B) It turns blue litmus red and smells like burning sulphur
(C) It turns res litmus blue and smells like vinegar
(D) It turns red litmus blue and has a fruity smell
What type of compound is formed by the reaction between acetic acid and an alcohol?
A few drops of ethanoic acid were added to solid sodium carbonate. The observation made was that ______.
Which of these is not an organic acid?
Ester is formed by the reaction between ______.
