Advertisements
Advertisements
प्रश्न
How is ethanoic acid obtained from ethanol? Write down the chemical equation of the reaction involved.
उत्तर
When ethanol (ethyl alcohol) is heated in the presence of alkaline potassium permanganate solution or acidified potassium dichromate solution, it gets oxidised to ethanoic acid. This reaction is known as oxidation reaction.
The chemical equation for the above reaction is:
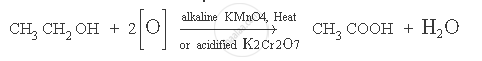
APPEARS IN
संबंधित प्रश्न
What is the common name of methanol?
Fill in the following blank with a suitable word:
The organic acid present in vinegar is ______.
The substance which can produce brisk effervescence with baking soda solution is:
(a) ethanol
(b) vegetable oil
(c) vinegar
(d) soap solution(b) hydrocarbon ends directed towards the centre and ionic ends directed outwards
In a soap micelle, the soap molecules are arranged radially with the hydrocarbon ends, i.e. hydrophobic, directed towards the centre; and, ionic ends, i.e. hydrophilic, directed outwards.
What is glacial acetic acid ?
A student is studying the properties of acetic acid in his school laboratory. List two physical and two chemical properties which he must observe and note in his record book.
A student puts a drop of acetic acid first on a blue litmus paper and then on a red litmus paper. He would observe that
(A) the red litmus paper turns colourless and there is no change in the blue litmus paper.
(B) the red litmus paper turns blue and the blue litmus paper turns red.
(C) there is no change in the red litmus paper and the blue litmus paper turns red.
(D) there is no change in the blue litmus paper and the red litmus paper turns blue.
State the observation
When the gaseous product obtained by dehydration of ethyl alcohol is passed through bromine water.
Ester is a sweet-smelling compound.
Which of these is not an organic acid?
Which of the following substance produces brisk effervescence with baking soda solution?
