Advertisements
Advertisements
प्रश्न
Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
पर्याय
pH of acetic acid is more than that of hydrochloric acid.
pH of acetic acid is less than that of hydrochloric acid.
Acetic acid dissociates completely in aqueous solution.
Acetic acid is a strong acid.
उत्तर
pH of acetic acid is more than that of hydrochloric acid.
APPEARS IN
संबंधित प्रश्न
You have four test tubes, A, B, C and D containing sodium carbonate, sodium chloride, lime water and blue litmus solutions respectively. Out of these the material of which test tube/ test tubes would be suitable for the correct test of acetic/ethanoic acid?
(a) only A
(b) A and B
(c) B and C
(d) A and D
Give balanced chemical equations for Sodium ethanoate to methane.
Name the following:
The distinctive reaction that takes place when ethanol is treated with acetic acid.
Which of the following will give brisk effervescence with sodium hydrogen carbonate and why?
CH3COOH, CH3CH2OH
Esters are sweet-smelling substances and are used in making perfumes. Describe an activity for the preparation of an ester with the help of a well labelled diagram. Write an equation for the chemical reaction involved in the formation of the ester. Also write the names of all the substances involved in the process of esterification.
Give the structural formulae of acetic acid.
Which of the following substance produces brisk effervescence with baking soda solution?
The reaction between acetic acid and sodium carbonate is shown in the following figure.
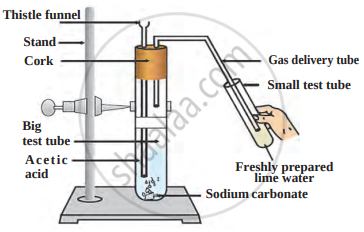
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
