Advertisements
Advertisements
प्रश्न
The reaction between acetic acid and sodium carbonate is shown in the following figure.
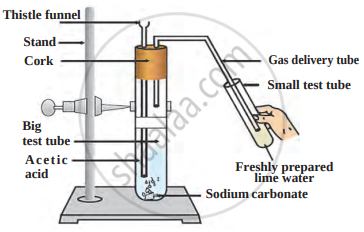
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
उत्तर
- Carbon dioxide gas.
- Lime water turns milky.
- \[\ce{2CH3COOH (aq) + Na2CO3 (g) -> CH3COONa (aq) + H2O (l) + CO2 (g)}\]
APPEARS IN
संबंधित प्रश्न
What do you observe when you drop a few drops of acetic acid to test tubes containing
(a) phenolphthalein
(b) distilled water
(c) universal indicator
(d) sodium hydrogen carbonate powder
Give balanced chemical equations for Sodium ethanoate to methane.
Give the common name and IUPAC name of C2H5OH.
Three organic compounds A, B and C have the following molecular formulae: C4H10O
Which compound contains a carboxyl group? Write its name and structural formula.
Vinegar is a solution of about:
(a) 5 to 8 per cent ethanoic acid in alcohol
(b) 5 to 8 per cent ethanoic acid in water
(c) 50 to 80 per cent ethanoic acid in water
(d) 50 to 80 per cent ethanoic acid in alcohol
Write the name of the first three members of the carboxylic acid series.
What do you notice when acetic acid reacts with litmus?
What is the main constituent of vinegar?
Fill in the blank with appropriate word/words.
Vinegar is a solution of about ________per cent ________in water.
Write a balanced equation for the following:
Write the equation for the preparation of ethylene from ethyl alcohol.
Draw the structural formula of a compound with two carbon atoms in the following case:
An alcohol containing two carbon atoms.
State how the following conversions can be carried out:
Ethyl chloride to Ethyl alcohol
State are relevant observations for following reactant:
Addition of ethyl alcohol to acetic acid in presence of conc. H2SO4
Explain the following reaction with an example.
Saponification
Which of the following substance produces brisk effervescence with baking soda solution?
Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
What happens when a small piece of sodium is dropped in ethanol? Write the equation for this reactions.
Raina while doing certain reactions observed that heating of substance ‘X’ with a vinegar-like smell with a substance ‘Y’ (which is used as an industrial solvent.) in the presence of conc. Sulphuric acid in a water bath gives a sweet-smelling liquid ‘Z’ having molecular formula C4H8O2. When heated with caustic soda (NaOH), ‘Z’ gives back the sodium salt and the compound ‘Y’.
Identify ‘X’, ‘Y’, and ‘Z’. Illustrate the changes with the help of suitable chemical equations.
Name the oxidising agent used in the conversion of ethanol to ethanoic acid.
