Advertisements
Advertisements
Question
What happens when ethanol reacts with ethanoic acid in the presence of a little of concentrated sulphuric acid? Write equation of the reaction involved.
Solution
When ethanol reacts with ethanoic acid in the presence of a few drops of concentrated sulphuric acid, a sweet smelling ester named ethyl ethanoate is formed.
The chemical equation for the above reaction is:
`CH_3 COOH+_2 H_5 OH` 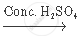 `CH_3 COOC_2 H_5 + H_2 O`
`CH_3 COOC_2 H_5 + H_2 O`
APPEARS IN
RELATED QUESTIONS
A carbon compound 'P' on heating with excess conc. H2SO4 forms another carbon compound 'Q' which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound 'R'. One molecule of 'R' on combustion forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved
Name the compound formed when ethanol is heated in excess of conc. sulphuric acid at 443 K. Also, write the chemical equation of the reaction stating the role of conc. sulphuric acid in it. What would happen if hydrogen is added to the product of this reaction in the presence of a catalyst such as palladium or nickel?
Match the formulae in group A with appropriate names from group B:
Group A: CH3COOH, CH3CHO, CH3OH
Group B: Ethanol, Methanol, Ethanal, Ethanoic acid
Name the product formed when ethanol reacts with acetic acid. Give an equation. What is the name given to this type of reaction?
How is ethane prepared by Wurtz reaction?
Write the equations of chlorination of ethane
Write a balanced chemical equation for the following:
Ethanol reacts with sodium at room temperature
Rectified spirit is an aqueous solution that contains about ______ of ethanol.
Give the balanced chemical equation of the following reaction:
Oxidation of ethanol by acidified potassium dichromate.
Give the balanced chemical equation of the following reaction:
Oxidation of ethanol by acidified potassium dichromate.
