Advertisements
Advertisements
Question
Name the compound formed when ethanol is heated in excess of conc. sulphuric acid at 443 K. Also, write the chemical equation of the reaction stating the role of conc. sulphuric acid in it. What would happen if hydrogen is added to the product of this reaction in the presence of a catalyst such as palladium or nickel?
Solution
The compound that is formed when ethanol is heated in excess of conc. sulphuric acid at 443 K is ethene.
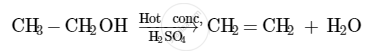
The concentrated sulphuric acid acts as a dehydrating agent in this reaction. It facilitates the removal of a molecule of water from the ethanol molecule.
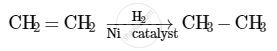
When hydrogen is added to ethene in the presence of a catalyst such as palladium or nickel, saturation of the compound takes place to form ethane.
APPEARS IN
RELATED QUESTIONS
Write the name and structural formula of the compound obtained when ethanol is heated at 443 K with excess of conc. H2SO4. Also write chemical equation for the reaction stating the role of conc. H2SO4 in it.
Organic compounds having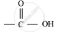 functional group are known as
functional group are known as
Describe one reaction of ethanol.
How would you test for an alcohol?
What happens when ethanol reacts with ethanoic acid in the presence of a little of concentrated sulphuric acid? Write equation of the reaction involved.
In a tabular form, differentiate between ethanol and ethanoic acid under the following heads:
(i) Physical state
(ii) Taste
(iii) NaHCO3 test
(iv) Ester test
The organic compound obtained as the end product of the fermentation of a sugar solution is ______.
Ethanol is soluble in water in all proportions.
100% pure ethanol is called ______.
What is denatured alcohol?
