Advertisements
Advertisements
Question
When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:-
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in the reaction.
Solution
(i). An ester is formed when an alcohol reacts with a carboxylic acid in the presence of an acidic medium. An ester has a fruity smell.
The chemical equation for the reaction between ethanol and ethanoic acid in the presence of conc. H2SO4 can be written as follows:-
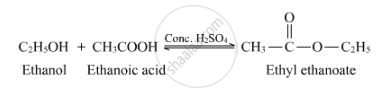
(ii). Concentrated sulphuric acid acts as a protonating catalyst during the esterification reaction.
RELATED QUESTIONS
When you add a few drops of acetic acid to a test-tube containing sodium bicarbonate powder, which one of the following is your observation?
(A) No reaction takes place
(B) A colourless gas with pungent smell is released with brisk effervescence
(C) A brown coloured gas is released with brisk effervescence
(D) Formation of bubbles of a colourless and odourless gas
Identify the term or substance based on the descriptions given below
Ice like crystals formed on cooling an organic acid sufficiently.
Draw the structures for the following compounds of Propanoic acid.
Give the name and structural formula of one homologue of HCOOH.
How would you distinguish experimentally between an alcohol and carboxylic acid on the basis of a chemical property?
The substance which can produce brisk effervescence with baking soda solution is:
(a) ethanol
(b) vegetable oil
(c) vinegar
(d) soap solution(b) hydrocarbon ends directed towards the centre and ionic ends directed outwards
In a soap micelle, the soap molecules are arranged radially with the hydrocarbon ends, i.e. hydrophobic, directed towards the centre; and, ionic ends, i.e. hydrophilic, directed outwards.
Give two tests to show that CH3COOH is acidic in nature ?
Write a balanced chemical equation for the following:
A mixture of sodalime and sodium acetate is heated.
Mineral acids are stronger acids than carboxylic acids because
- mineral acids are completely ionised
- carboxylic acids are completely ionised
- mineral acids are partially ionised
- carboxylic acids are partially ionised
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
