Advertisements
Advertisements
प्रश्न
When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:-
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in the reaction.
उत्तर
(i). An ester is formed when an alcohol reacts with a carboxylic acid in the presence of an acidic medium. An ester has a fruity smell.
The chemical equation for the reaction between ethanol and ethanoic acid in the presence of conc. H2SO4 can be written as follows:-
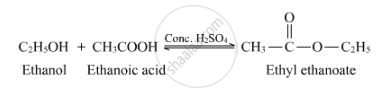
(ii). Concentrated sulphuric acid acts as a protonating catalyst during the esterification reaction.
संबंधित प्रश्न
Complete the following chemical equations: C2H5OH+O2→
An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogen carbonate. Give the name and formula of X.
What do you observe when acetic acid is added to a neutral FeCl3 solution?
Name the functional group present in the following compound:
C2H5CHO
Which one of the following are the correct observations about acetic acid?
(A) It turns blue litmus red and smells like vinegar
(B) It turns blue litmus red and smells like burning sulphur
(C) It turns res litmus blue and smells like vinegar
(D) It turns red litmus blue and has a fruity smell
Fill in the blank with appropriate word/words.
Esterification is the reaction between carboxylic acid and ______ in presence of ____
Draw the structural formula of a compound with two carbon atoms in the following case:
An alkane with a carbon to carbon single bond.
CH3–CH2–CHO : propanal : : CH3–COOH : _______
Ethanoic acid reacts with the basic salt, sodium carbonate, to form a salt, named ______, water and carbon dioxide gas.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
