Advertisements
Advertisements
प्रश्न
What do you observe when acetic acid is added to a neutral FeCl3 solution?
उत्तर
When acetic acid is added to neutral FeCl3, wine red color is produced.
APPEARS IN
संबंधित प्रश्न
Write three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate. Write balanced chemical equation in each case. Write the name of the reactants and the products other ethanoic acid and sodium ethanoate in each case.
In order to study saponification reaction, we first prepare 20% solution of sodium hydroxide. If we record the temperature of this solution just after adding sodium hydroxide flakes to water and also test its nature using litmus, it may be concluded that the process of making this solution is
(A) exothermic and the solution is alkaline
(B) endothermic and the solution is alkaline
(C) endothermic and the solution is acidic
(D) exothermic and the solution is acidic
Write the name and chemical formula of the simplest organic acid.
Name the products formed and give appropriate chemical equations for the following:
sodium reacting with ethyl alcohol.
Which one of the following are the correct observations about acetic acid?
(A) It turns blue litmus red and smells like vinegar
(B) It turns blue litmus red and smells like burning sulphur
(C) It turns res litmus blue and smells like vinegar
(D) It turns red litmus blue and has a fruity smell
Acetic acid solution turns:
(1) blue litmus red
(2) red litmus blue
(3) blue litmus colourless
(4) red litmus colourless
Write a balanced equation for the following:
Write the equation for the preparation of ethylene from ethyl alcohol.
Draw the structure formula of ethyne.
Which of the following substance produces brisk effervescence with baking soda solution?
Observe the diagram given below and answer the questions:
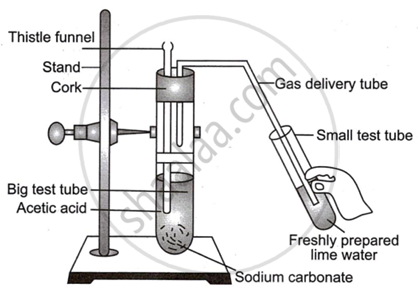
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
