Advertisements
Advertisements
प्रश्न
When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:-
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in the reaction.
उत्तर
(i). An ester is formed when an alcohol reacts with a carboxylic acid in the presence of an acidic medium. An ester has a fruity smell.
The chemical equation for the reaction between ethanol and ethanoic acid in the presence of conc. H2SO4 can be written as follows:-
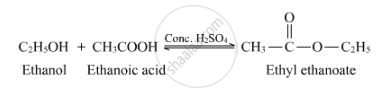
(ii). Concentrated sulphuric acid acts as a protonating catalyst during the esterification reaction.
संबंधित प्रश्न
Complete the following chemical equations :CH3COOH+NaOH→
What happens when propanoic acid is warmed with methanol in the presence of a few drops of concentrated sulphuric acid? Write equation of the reaction involved.
What is the physical state of CH3COOH?
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is a constituent of vinegar?(e) Which compound is a constituent of vinegar?
What do you notice when acetic acid reacts with metals?
Choose the correct word/phrase from the options given below to complete the following sentence:
When acetaldehyde is oxidized with acidified potassium dichromate, it forms ______.
Choose the correct option from given alternative:
When sodium hydrogen carbonate is added to ethanoic acid a gas evolves. Consider the following statements about the gas evolved?
(A) It turns lime water milky.
(B) It is evolved with brisk effervescence.
(C) It has a smell of burning sulfur.
(D) It is also a by-product of respiration.
Give the balanced chemical equation of the following reaction:
Evolution of carbon dioxide by the action of ethanoic acid with NaHCO3.
A few drops of ethanoic acid were added to solid sodium carbonate. The observation made was that ______.
Which of these is not an organic acid?
