Advertisements
Advertisements
प्रश्न
What happens when ethanol reacts with ethanoic acid in the presence of a little of concentrated sulphuric acid? Write equation of the reaction involved.
उत्तर
When ethanol reacts with ethanoic acid in the presence of a few drops of concentrated sulphuric acid, a sweet smelling ester named ethyl ethanoate is formed.
The chemical equation for the above reaction is:
`CH_3 COOH+_2 H_5 OH` 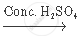 `CH_3 COOC_2 H_5 + H_2 O`
`CH_3 COOC_2 H_5 + H_2 O`
APPEARS IN
संबंधित प्रश्न
How would you distinguish experimentally between an alcohol and a carboxylic acid?
Write the molecular formula of ethanol.
Explain why, methanol is much more dangerous to drink than ethanol.
When ethanol reacts with sodium metal, it forms two products. These products are:
(a) sodium ethanaoate and oxygen
(b) sodium ethanaoate and hydrogen
(c) sodium ethoxide and oxygen
(d) sodium ethoxide and hydrogen
State the method of preparation of ethanol by hydrolysis of ethyl bromide.
How is the absolute alcohol obtained?
Write a balanced chemical equation for the following:
Ethanol under high pressure and low temperature is treated with acidified potassium dichoromate.
[Ethane, Ethene, Ethanoic acid, Ethyne, Ethanol]
From the above list, name the compound with \[\ce{-OH}\] as the part of its structure.
100% pure ethanol is called ______.
Give the balanced chemical equation of the following reaction:
Oxidation of ethanol by acidified potassium dichromate.
