Advertisements
Advertisements
प्रश्न
What happens when ethanol reacts with ethanoic acid in the presence of a little of concentrated sulphuric acid? Write equation of the reaction involved.
उत्तर
When ethanol reacts with ethanoic acid in the presence of a few drops of concentrated sulphuric acid, a sweet smelling ester named ethyl ethanoate is formed.
The chemical equation for the above reaction is:
`CH_3 COOH+_2 H_5 OH` 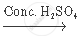 `CH_3 COOC_2 H_5 + H_2 O`
`CH_3 COOC_2 H_5 + H_2 O`
APPEARS IN
संबंधित प्रश्न
A carbon compound 'P' on heating with excess conc. H2SO4 forms another carbon compound 'Q' which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound 'R'. One molecule of 'R' on combustion forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved
Write balanced chemical equations for Preparation of ethanol from Ethyl Chloride
Write the molecular formula of ethanol.
State two used of ethanol (other than as a fuel).
State the method of preparation of ethanol by hydrolysis of ethyl bromide.
how can we prepare ethane by reduction of a halogen compound?
Write the rnolecular formula and structural forrnula of ethanol.
Choose the correct alternative and rewrite the following:
To observe the hydro and clearly, Raju should see it first under the low power lens and then under the high power lens in order to see _____________
What is denatured alcohol?
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
