Advertisements
Advertisements
Question
A carbon compound 'P' on heating with excess conc. H2SO4 forms another carbon compound 'Q' which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound 'R'. One molecule of 'R' on combustion forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved
Solution

APPEARS IN
RELATED QUESTIONS
Write a balanced chemical equations for Heating of Ethanol at 170°C in the presence of conc. Sulphuric acid
What is meant by a functional group? Explain with an example.
Complete the following equation:
`CH_3 CH_2 OH` 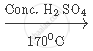
What happens when ethanol is oxidised with alkaline potassium permanganate (or acidified potassium dichromate)? Write the equation of the reaction involved.
Write a chemical reaction to show the dehydration of ethanol.
Write the equations of chlorination of ethane
Write the characteristics of ethanol.
C2H5OH + 3O2 → 2CO2 + 3H2O is a ______
How is ethanol manufactured from sugarcane?
Give the balanced chemical equation of the following reaction:
Combustion of ethanol.
