Advertisements
Advertisements
Question
Write names and formulae of hydrocarbons containing a single and a double bond (one example for each). Give one characteristic chemical property of each.
Solution
Methane is a hydrocarbon which has a single bond. Its formula is CH4.
The characteristic chemical property of methane is the substitution reaction. It is a reaction in which one or more hydrogen atoms are replaced by some other atoms.
Chemical equation:
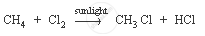
Ethene is a hydrocarbon containing a double bond. Its formula is C2H4.
The characteristic chemical property of ethene is the addition reaction. It is a reaction in which unsaturated compound (ethene) is combined with another substance to form a single product (saturated compound).
Chemical equation:
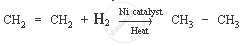
APPEARS IN
RELATED QUESTIONS
Give a balanced chemical equation for Action of alcoholic KOH on bromethane
Why is ethanol used as a fuel?
Give the harmful effects of drinking alcohol.
When ethanol reacts with sodium metal, it forms two products. These products are:
(a) sodium ethanaoate and oxygen
(b) sodium ethanaoate and hydrogen
(c) sodium ethoxide and oxygen
(d) sodium ethoxide and hydrogen
Give the necessary conditions and equations of getting ethanol from:
(a) Alkyl halide
(b) An ethene
Which of the following are used as anesthetics?
Dehydration of ethanol by conc. Sulphuric acid forms ______
100% pure ethanol is called ______.
Write the chemical equation for the following:
Oxidation of ethanol
What is the role of concentrated Sulphuric acid when it is heated with Ethanol at 443 K. Give the reaction involved.
