Advertisements
Advertisements
Question
What is the molecular formula and structural formula of a cyclic hydrocarbon whose one molecule contains 8 hydrogen atoms?
Solution
The molecular formula of a cyclic hydrocarbon is C4H8, as it satisfies the general formula of a cyclic hydrocarbon, i.e. CnH2n. Here, the number of hydrogen atoms is already given as 8, i.e. 2n = 8.
Therefore, n = 8/2 = 4. Here, n represents the number of carbon atoms. Therefore, the molecular formula is C4H8.
The structural formula of the cyclic hydrocarbon C4H8 is as follows:
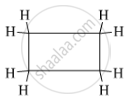
APPEARS IN
RELATED QUESTIONS
Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential condition for an addition reaction. Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction.
Define the term ‘structural’ isomerism'.
Draw the structures for Bromopentane*
Draw the structures of possible isomers of butane, C4H10.
Give reason why the carbon compounds generally have low melting and boiling points.
Give reason why the carbon compounds do not conduct electricity in the molten state.
Write the molecular formula and structure of benzene.
The number of carbon atoms in the organic compound named as 2,2-dimethylpropane is:
(a) two
(b) five
(c) three
(d) four
A reagent which can help us to distinguish between alkenes and alkynes is ______.
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
