Advertisements
Advertisements
प्रश्न
What is the molecular formula and structural formula of a cyclic hydrocarbon whose one molecule contains 8 hydrogen atoms?
उत्तर
The molecular formula of a cyclic hydrocarbon is C4H8, as it satisfies the general formula of a cyclic hydrocarbon, i.e. CnH2n. Here, the number of hydrogen atoms is already given as 8, i.e. 2n = 8.
Therefore, n = 8/2 = 4. Here, n represents the number of carbon atoms. Therefore, the molecular formula is C4H8.
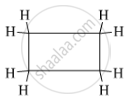
The structural formula of the cyclic hydrocarbon C4H8 is as follows:
APPEARS IN
संबंधित प्रश्न
Draw the structures for Bromopentane*
Fill in the following blank with suitable word:
CnH2n is the general formula of .......... hydrocarbons.
Write the names and structural formulae of all the possible isomers of hexane.
Which of the following is the molecular formula of benzene?
C6H6, C6H10, C6H12, C6H14
What is the unique property of carbon atom? How is this property helpful to us?
The pair of elements which exhibits the property of catenation is:
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains single bonds as well as a double bond?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae represent open chain unsaturated hydrocarbons having double bonds?
Match the reactions given in Column (A) with the names given in column (B).
| Column (A) | Column (B) | ||
(a) |
`"CH"_3"OH" + "CH"_3"COOH"overset("H"^+)(->) "CH"_3"COOCH"_3 + "H"_2"O"` | (i) | Addition reaction |
| (b) | `"CH"_3 = "CH"_2 + "H"_2 overset("Ni")(->)"CH"_3 - "CH"_3` | (ii) | Substitution reaction |
| (c) | `"CH"_4 + "Cl"_2overset("Sunlight")(->)"CH"_3"Cl" + "HCl"` | (iii) | Neutralisation reaction |
| (d) | `"CH"_3"COOH" + "NaOH" -> "CH"_3"COONa" + "H"_2"O"` | (iv) | Esterification reaction |
Recognise the carbon chain type of the following:
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\\ce{H - C - C - C - C - H}\\|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}
\end{array}\]
