Advertisements
Advertisements
प्रश्न
Give one example of a decomposition reaction which is carried out by applying heat.
उत्तर
Example of the decomposition reaction are as follows:
by applying heat:
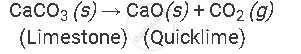
APPEARS IN
संबंधित प्रश्न
Write one equation for decomposition reactions where energy is supplied in the form of heat.
Taking into consideration the relationship in the first pair, complete the second pair
2H2 + O2 → 2H2O :Combination Reaction :: 2HgO → 2Hg + O2:_________
What type of reaction is represented by the following equation?
NH4 CI → NH3 + HCI
Differentiate between direct combination reaction and a decomposition reaction.
Give a balanced equation for –
A double decomposition neutralization reaction involving an acid and a base
Differentiate between the following:
Thermal decomposition and thermal dissociation.
Differentiate between the following:
Electrolytic decomposition and photochemical decomposition.
The chemical reaction in which two or more products are formed from a single reactant is called _______ reaction.
On heating blue coloured powder of copper (II) nitrate, in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed
- Write a balanced chemical equation of the reaction.
- Identify the brown gas X evolved.
- Identify the type of reaction.
- What could be the pH range of aqueous solution of the gas X?
When lead nitrate is heated strongly in a boiling tube, two gases are liberated and a solid residue is left behind in the test tube.
- Name the type of chemical reaction and define it.
- Write the name and formula of the coloured gas liberated.
- Write the balanced chemical equation for the reaction.
- Name the residue left in the test tube and state the method of testing its nature (acidic/basic).
