Advertisements
Advertisements
प्रश्न
Write one equation for decomposition reactions where energy is supplied in the form of heat.
उत्तर
Decomposition reaction involving absorption of heat,
- \[\ce{\underset{Zinc carbonate}{ZnCO3_{(s)}} ->[\Delta]\underset{Zinc oxide}{ZnO_{(s)}} + \underset{Carbon dioxide}{CO2_{(g)}}}\]
- \[\ce{CaCO3->[heat]CaO_{(s)} + CO2_{(g)}}\]
- \[\ce{2FeSO4(s) ->[heat] Fe2O3(s) + SO2(g) + SO3(g)}\]
APPEARS IN
संबंधित प्रश्न
What does one mean by exothermic reaction? Give example.
What type of reaction is represented by the digestion of food in our body?
Give one example of a decomposition reaction which is carried out with electricity.
What type of chemical reaction take place when electricity is passed through water?
What type of chemical reaction is represented by the following equation?
X → Y + Z
What type of reaction is represented by the following equation?
NH4NO2 → N2 + 2H2O
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction?
What are thermal decomposition reactions ? Explain with an example.
What does one mean by endothermic reaction? Give example.
Classify the following reaction into different type:
\[\ce{2KClO3(s)->[\Delta] 2KCl(aq) + 3O2(g)}\]
Give a balanced equation for –
A white precipitate obtained during a double decomposition reaction involving a silver salt with sodium salt.
Give the ratio in which hydrogen and oxygen are present in water by volume.
Which among the following statement(s) is (are) true?
Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride.
(ii) sublimation of silver chloride.
(iii) decomposition of chlorine gas from silver chloride.
(iv) oxidation of silver chloride.
When SO2 gas is passed through a saturated solution of H2S, which of the following reaction occurs?
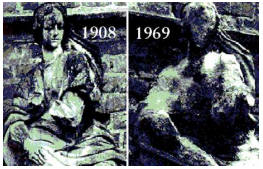
Marble statues are corroded or stained rain water. Identify the main reason.
The reaction of calcium carbonate, with freshly prepared lime water, is shown below:
Answer the questions with the help of a diagram:
- What type of reaction does calcium carbonate undergoes?
- What change in colour is observed in lime?
- Write the chemical equation.


- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
Complete the following reaction:
\[\ce{C_12H_22O11->[Heat]}\] ______ + ______.
When lead nitrate is heated strongly in a boiling tube, two gases are liberated and a solid residue is left behind in the test tube.
- Name the type of chemical reaction and define it.
- Write the name and formula of the coloured gas liberated.
- Write the balanced chemical equation for the reaction.
- Name the residue left in the test tube and state the method of testing its nature (acidic/basic).
